Carbon-14 Decays to Nitrogen by Which of the Following Processes
Atmospheric 14 C is produced from the interaction of 14 N and secondary cosmic ray neutrons by the following exothermic nuclear reaction. 1 emission of electron electron capture.

Learn About Beta Decay Of Carbon 14 Chegg Com
1b 10 24 cm 2.
. The decay of a carbon-14 atom inside DNA in one person happens about 50 times per second changing a carbon atom to one of nitrogen. Heres a video to give more explanation. Carbon-14 has a half-life disintegration.
It has a half - life of nearly 6000 years so by measuring the relative amount of carbon-14 in a bone archeologists can calculate when the. When the biological system dies it stops exchanging carbon. An electro in innershell of atom combines with proton forming neutr.
What would happen to the molecule when the atom decays into nitrogen. Once the organism dies however it ceases to absorb carbon-14 so that the amount of the isotope in its tissues steadily decreases. When carbon-14 decays to form nitrogen-14 the carbon nucleus emits an __.
Carbon-14 dating works for fossils as old as 50000 years. Carbon 14 146 C has six protons in its nucleus and is formed in our atmosphere by cosmic ray bombardment of nitrogen 14 147 N. When carbon-14 decays into nitrogen-14 what happens is the following.
The electron and the anti-neutrino are emitted from the nucleus and the nucleus is left with. Carbon-14 dating is used to date very old volcanic rock. Carbon-14 is radioactive with a half-life of about 5700 years.
A carbon-14 nucleus decays to a nitrogen-14 nucleus by beta decay. You can rearrange this equation to solve for t by following these steps. The annual dose from carbon-14 is estimated to be about 12 μSvyear.
Which of the following is a radioisotope commonly used in dating archeological artifacts. Will it be converted into a ceNO2 molecule or will it split apart. For the decay of carbon-14 to nitrogen-14 k 000012097.
View the full answer. A reducing the size of certain tumors generating radiation for medical imaging C producing energy in nuclear power plants determining the age of buried organic materials. Carbon-14 can also be produced in the atmosphere by other neutron reactions including in particular 13Cnγ14C and 17Onα14C.
The decay that causes this change is called radioactive decay. Take the natural logarithm of both sides of the equation. Carbon-14 decays into nitrogen-14.
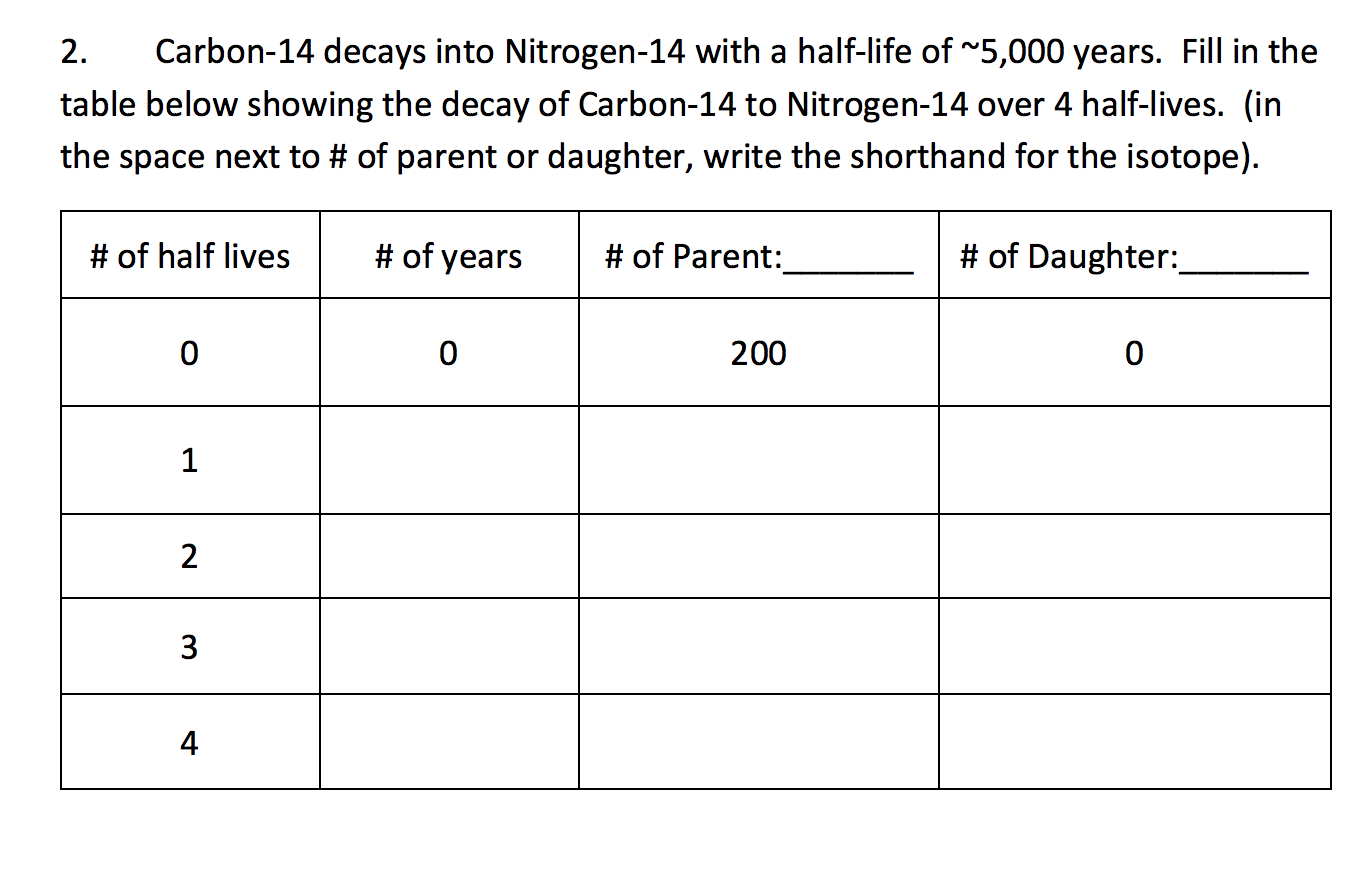
Carbon-14 6 protons and 8 neutrons decays into nitrogen-14 7 protons and 7 neutrons through beta decay. Physics questions and answers. Carbon-14 C-14 is a radioactive isotope with a half-life of 5730 years.
Carbon-14 decays by beta- which emits a W- boson thatimmediately decays into an electron and an electronanti-neutrino. A neutron in the atom undergoes decay and will produce a proton electron the beta particle and an electron antineutrino. Carbon-14 dating also called radiocarbon dating method of age determination that depends upon the decay to nitrogen of radiocarbon carbon-14.
Carbon-14 has a longer half-life than potassium-40. Rewrite the right side of this equation by applying the following rule. The neutrons required for this reaction are produced by cosmic rays interacting with the atmosphere.
Lead-206 to bismuth-166 argon-40 to potassium-40 carbon-14 to carbon-12 carbon-14 to nitrogen-14 potassium-40 to argon-40. Slowly the carbon-14 atoms decay to nitrogen without being replaced so that there is less and less carbon-14 in a dead body. Carbon -14 is continually formed in nature by the interaction of neutrons with nitrogen-14 in the Earths atmosphere.
It decays to stable nitrogen-14 N-14 through beta decay. Carbon 14 is unstable and undergoes radioactive decay by emitting a beta particle an electron and an antineutrino zero mass zero charge and becoming nitrogen seven protons. Carbon-14 decays slowly in a living organism and the amount lost is continually replenished as long as the organism takes in air or food.
As a result carbon-14 is continuously formed in the upper atmosphere by the interaction of cosmic rays with. Select all that apply beta decay electron capture alpha decay positron emission Submit Answer Retry Entire Group No. The rate at which carbon-14 decays is constant and follows first order kinetics.
When she returned to analyze it 6 days later only 625 g. N 14 n C 14 p 062 M e V σ 181 b where the cross section σ is expressed in barnb. Since lne 1 simplify the equation.
One of the neutrons decays into a proton an electron an anti-neutrino. These neutrons produced in these collisions can be absorbed by nitrogen-14 to produce an isotope of carbon-14. Once a living thing dies it no longer takes in carbon.
The half-life of carbon-14 is 5730 years but carbon-14 does not change. Which process is a current use of C-14. When the neutron collides a nitrogen-14 seven protons seven neutrons atom turns into a carbon-14 atom six protons eight neutrons and a hydrogen atom one proton zero neutrons.
When the nuclide carbon-14 decays to nitrogen-14 what kind of decay does carbon-14 undergo. Which of the following are radioactive decay processes used to determine the age of samples. A scientist studying an unusual radioisotope had a 500 g sample of the substance in her lab.
Lnex x lne. As long as the biological system is alive the level is constant due to constant intake of all isotopes of carbon. See answer 1 Best Answer.
LnF lne -kt. 00157 MeV 0157 MeV. The half-life of carbon-14 is 13 billion years and carbon-14 decays into nitrogen-14.
How much energy in MeV is released if carbon-14 has a mass of 14003242 u and nitrogen-14 has a mass of 14003074 u. C-14 - N-14 beta- The carbon-14 atoms undergo beta-minus decay electron emission and produce a beta particle and a nitrogen-14 atom. In nuclear physics beta decay β-decay is a type of radioactive decay in which a beta ray fast energetic electron or positron and a neutrino are emitted from an atomic nucleus.
In this process a neutral particle in the l4 C nucleus called a neutron. 8595 years 2865 years 5730 years 11460 years 1433 years Select the TWO answers that are correct.

21 3 Radioactive Decay Chemistry
Solved 2 Carbon 14 Decays Into Nitrogen 14 With A Half Life Chegg Com
Solved 2 Carbon 14 Decays Into Nitrogen 14 With A Half Life Chegg Com

Solved When The Nuclide Carbon 14 Decays To Nitrogen 14 Chegg Com
Comments
Post a Comment